Zhiteng Chen, Jingqun Ao, Wenchuan Yang, Liping Jiao, Tianling Zheng, Xinhua Chen,Appl Microbiol Biotechnol, 2013. 97:10381-10390
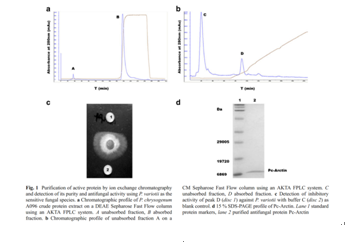
A fungal strain,Penicillium chrysogenumA096,was isolated from an Arctic sediment sample. Its culture supernatant inhibited mycelial growth of some plant pathogenicfungi. After saturation ofP. chrysogenumA096 culture supernatant with ammonium sulfate and ion exchange chromatography, a novel antifungal protein (Pc-Arctin) was purified andidentified by matrix assisted laser desorption ionization-time offlight-time of flight-mass spectrometry (MALDI-TOF-TOF-MS). The gene encoding for Pc-Arctin consisting of 195 nucleotides was cloned from P. chrysogenum A096 to confirm themass spectrometry result. Pc-Arctin displays antifungal activityagainstPaecilomyces variotii,Alternaria longipes, andTrichoderma virideat minimum inhibitory concentrations(MIC) of 24, 48, and 192 ng/disc, respectively. Pc-Arctin wasmost sensitive to proteinase K and then to trypsin but insensitive to papain. Pc-Arctin possesses high thermostability andcannot be antagonized by common surfactants, except forsodium dodecyl sulfate (SDS). Divalent ions, such as Mn2+,Mg2+, and Zn2+, inhibited the antifungal activity of Pc-Arctin.Hemagglutination assays showed that Pc-Arctin had nohemagglutinating or hemolytic activity against red blood cells(RBC) from rabbits, rats, and guinea pigs. Therefore, Pc-Arctinfrom ArcticP. chrysogenummay represent a novel antifungalprotein with potential for application in controlling plant pathogenic fungal infection.